CH310M/318M Organic I
|
Dr. Brian
Pagenkopf |
|
|
|
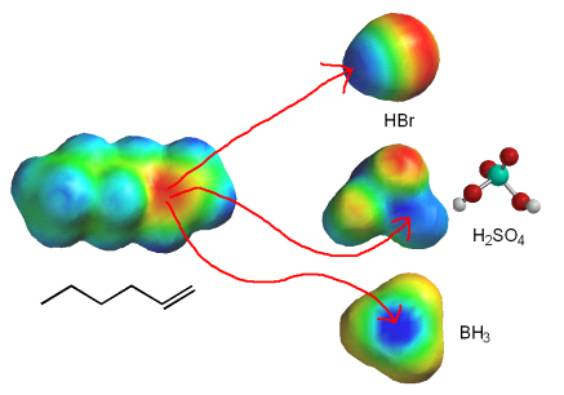
In the alkene reactions in Chapter 6, the alkene pi bond is the nucleophile and some other reagent is the electrophile.
In the reaction of alkenes with HCl there is a distinct intermediate carbocation formed, and by estimating the relative stabilities of the two one can predict products. We need to know details about the mechanism in order to make product predictions. The acid catalyzed addition of water (hydration) is fundamentally the same mechanism. Because the addition of HCl or H2O proceed through a carbocation intermediate the reaction can lose stereochemical information. The reaction is NOT stereospecific.
For the other alkene reactions we need to memorize what the mechanism is for each reaction. Once we remember the mechanism, we can predict products from any new substrate.
Addition of Br2
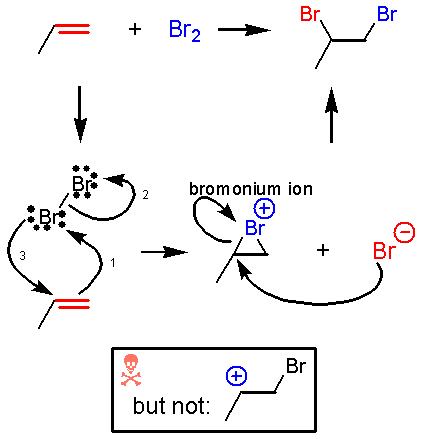
A lot happens in the first step of this reaction. The bromine-bromine bond is very weak. Attack by the alkene nucleophile (1) causes the bromine-bromine bond to break (2) so that bromine doesn’t end up with more than 8 valence electrons. Experiments suggest that there is no discrete carbocation intermediate, so the bromine must donate a pair of electrons to the other end of the alkene as it is being attacked (3). Instead of an intermediate cation, there is an intermediate bromonium ion that is then attacked by Br¯. The significance of an intermediate bromonium ion and not a carbocation is that the reaction is stereospecific.
The
fundamentals of this mechanism are the same as for halohydrin formation
(addition of Br and OH).
Hydroboration.
In the hydroboration reaction once again the alkene can be viewed as a nucleophile attacking an electrophilic boron atom. The hydroboration reaction is also stereospecific, and this tells us some information about the mechanism. Instead, the electron arrow pushing looks a lot like the addition of bromine. The alkene donates a pair of electrons to boron (1), and as it does this the hydrogens on boron become more hydride-like and the adjacent carbon gets more cationic character. As the charges build up on the adjacent carbon and on the hydrogen they start to make a bond at the same time (2). So, arrows one and two happen together. While attack of the boron by the alkene could make a carbocation (like the addition of H+) an intermediate carbocation like XX doesn’t form. This can be represented by a four membered transition state (XX).
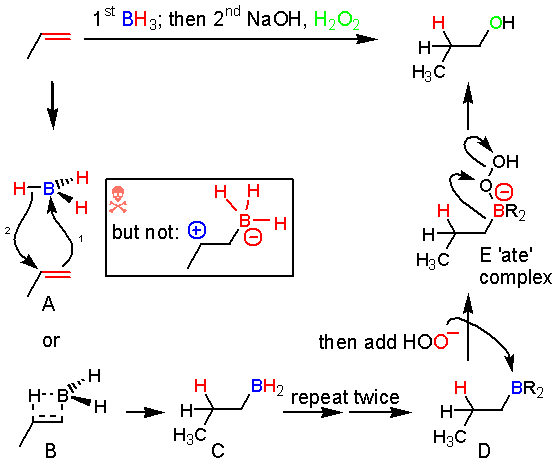
An interesting point about boron chemistry is that when boron has 8 electrons around it from donation of an electron pair by a Lewis base (together called a boron ‘ate’ complex), the bond between boron and the other groups, including hydrogen and carbon atoms, becomes much weaker and the hydrogen or carbon atoms can leave as hydrides (H:¯) or carbanions. This phenomenon explains the intermediate B and the second migration E. The oxygen-oxygen bond is very weak, just like the Br-Br or Cl-Cl bond.