CH310M/318M Organic I
|
Dr. Brian
Pagenkopf |
|
|
|
Hydroboration
/ Oxidation.
Borane (BH3) is a Lewis acid and can react with the electron rich alkene (a Lewis Base).
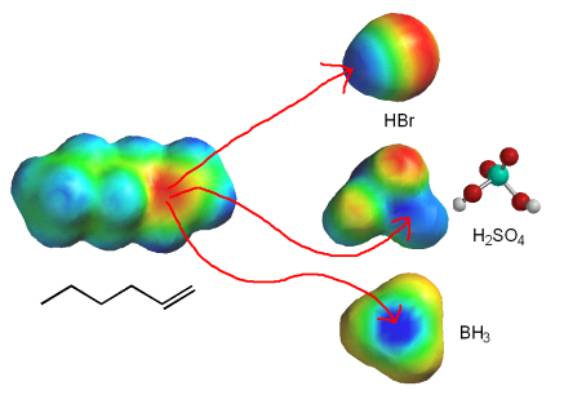
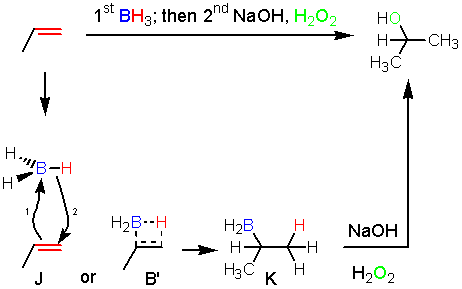
In the hydroboration reaction once again the alkene can be viewed as a nucleophile attacking an electrophilic boron atom. The hydroboration reaction is also stereospecific, and this tells us some information about the mechanism. Instead, the electron arrow pushing looks a lot like the addition of bromine. The alkene donates a pair of electrons to boron (1), and as it does this the hydrogens on boron become more hydride-like and the adjacent carbon gets more cationic character. As the charges build up on the adjacent carbon and on the hydrogen they start to make a bond at the same time (2). The electon movement shown by arrows one and two happens at exactly the same time. While attack of the boron by the alkene could make a carbocation (like the addition of H+) an intermediate carbocation like F doesn’t form. This can be represented by a four membered transition state (B). Note that the reaction gives the opposite result from hydration (H2SO4, H2O), so the reaction is anti-Markovnikov. However, mechanistically both hydration and hydroboration reactions are similar in that each involves attack by the alkene (the Lewis base) on a Lewis acid.

An interesting point about boron chemistry is that when boron has 8 electrons around it from donation of an electron pair by a Lewis base (together called a boron ‘ate’ complex), the bond between boron and the other groups, including hydrogen and carbon atoms, becomes much weaker. In a boron ate complex the hydrogen or carbon atoms can leave as hydrides (H:¯) or carbanions. This phenomenon helps explains the simultaneous bond breaking and bond making in intermediate B and the second migration event shown in E. In step E, it is helpful to know that the oxygen-oxygen bond is very weak, just like the Br-Br or Cl-Cl bond.
The hydroboration of propene gives 1-propanol (above) but not 2-propanol, which would be expected if the reaction went through intermediate B'.
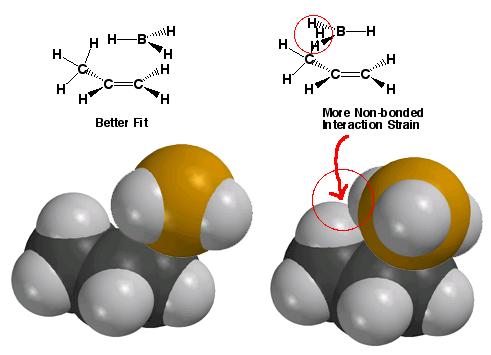
What’s the reason for the regioselectivity on the hydroboration reaction? Even though the hydrogens bonded to boron are very small, better orbital overlap and lower energy transition states are possible when the hydrogens on boron point away from the largest part of the alkene. Therefore, non-bonded interaction (or steric strain) are important in explaining the regiochemistry of the hydroboration reaction.