CH310M/318M Organic I
|
Dr. Brian
Pagenkopf |
|
|
|
Exam 3
Review. Chapters 7, 8 and 9.
Key Words: Allylic, anti-periplanar, Bi-molecular elimination, Bi-molecular nucleophilic substation, co-planar, E1, E2, Electrophile, H2CrO4, HOIO3, Hydrogen abstraction, Inversion of stereochemistry, Leaving group, Nucleophile, PCC, Pentacoordinate, Pinacol rearrangement, Radical halogenation, SN1, SN2, Sulfonate, Tosylate, Ts, Uni-molecular elimination, Uni-molecular nucleophilic substitution, b-elimination.
Halo alkanes are important compounds used as electrophiles in many kinds of reactions, and we need to know several ways of making them. In Chapter 7 the radical chlorination and bromination of saturated and unsaturated hydrocarbons was described. Bromination is much more selective than chlorination. Hydrogen abstraction occurs most easily with tertiary hydrogens, then secondary, and lastly, primary. A carbon centered radical is electron deficient and resembles a carbocation.
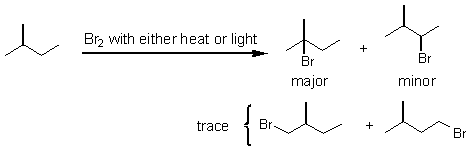
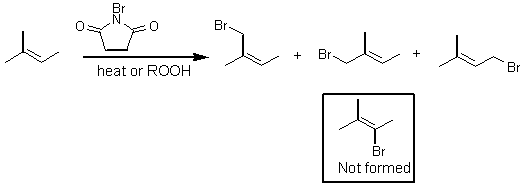
When a double bond is present under radical halogenation conditions, hydrogen abstraction occurs at the allylic position, and not at the double bond.
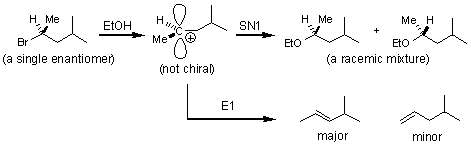
Chapter 8 showed that halo-alkanes are useful reagents for a variety of reactions. The four broadest categories of organic reactions are described as: SN2, SN1, E2 and E1, and these designations are based on the mechanism of the reaction. The number 1 or 2 is assigned after extensive study of the reaction kinetics, and 1 stands for unimolecular and 2 for bi-molecular. The SN part stands for nucleophilic substitution, and the E stands for elimination.
SN1 and E1 reactions are similar in that each involves the formation of an achiral carbocation intermediate. SN1 and E1 reactions are favored in polar protic solvents.
In an SN2 reaction the nucleophile attacks the carbon bearing the leaving group (LG) before the bond between the LG and carbon are broken. The result is an intermediate pentacoordinate carbon, with bond breaking and bond making between the leaving group and nucleophile, respectively, occurring at the same time. The stereochemical result of this mechanism is that addition always occurs with inversion of stereochemistry.
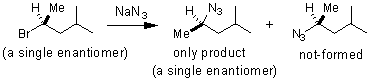
An E2 elimination reaction is also called a b-elimination reaction because there must be a b-hydrogen available for abstraction by a strong base. There’s also a stereochemical requirement for the abstraction: the b-hydrogen must be anti and coplanar to the leaving group (also called anti-periplanar). This stereochemical requirement becomes important when cyclic substrates are considered.
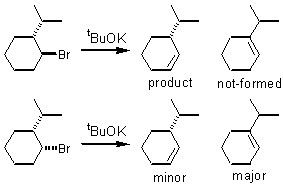
To predict reaction outcomes, you should be able to reproduce and understand the reactions decision tree.
Chapter 9 explored the chemistry of alcohols. The chapter is divided into three main sections. The first section explained that “HOŻ” is a terrible leaving group, so nucleophiles don’t displace alcohols. However, protonating the alcohol with strong acid allows it to leave as a neutral water molecule, “H2O”, and water is an excellent leaving group. Alcohols can also be converted into other leaving groups.
|
|
Reagent |
Makes… |
|
|
PBr3 |
Alkyl bromides, RBr |
|
|
SOCl2 |
Alkyl chlorides, RCl |
|
|
TsCl, py |
Alkyl sulfonates (tosylates, ROSO2Tol), ROTs |
Alkyl tosylates are excellent substrates for SN2 reactions, and most nucleophiles will displace them.
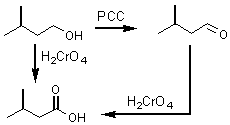
The second section of chapter 9 addressed the oxidation of alcohols to aldehydes, ketones or carboxylic acids. The two reagents used for these transformations are PCC and H2CrO4.
HOIO3 was shown to oxidize vicinal diols to two carbonyl groups. The stereochemical requirement for this oxidation is that the two alcohols are close enough together so that they can form a periodate ester, or 5 membered ring intermediate.
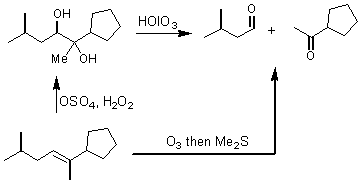
The third section of chapter 9 introduced the pinacol rearrangement. The mechanism of the pinacol rearrangement is very common and related reactions frequently occur in biological systems.