CH610B Sophomore Organic II
|
Dr. Brian Pagenkopf |
|
|
|
Carboxylic acids are acidic
because their anionic salts are stabilized by resonance (they’re really great
leaving groups).

When comparing acid strengths, it is usually helpful to compare the structures of the negatively-charged conjugate bases produced when the acids are deprotonated. When comparing similar acids, the more stable the conjugate base, the more acidic the parent acid. Mother Nature hates localized charges, so the more delocalized a charge, the more stable the ion. Thus, a more stable anionic conjugate base can usually be identified as the one that has the negative charge distributed over more atoms. This is a huge effect. The carboxylate anion (above right) has the negative charge (red color) distributed over both oxygen atoms, while the alkoxide anion (above left) has the negative charge (darker red color) localized on only one oxygen atom. Thus, it is no surprise that acetic acid is 12 orders of magnitude (roughly the size of the national debt in dollars!) more acidic than ethanol.
Increasing Acidity Due to Increased Delocalization of Negative Charge in the Carboxylate: The Inductive Effect
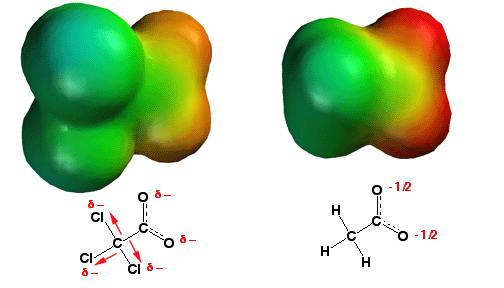
Any atom, group of atoms, or
functional gropus that can help delocalize the negative charge of a conjugate
base makes the parent acid more acidic. The electronegative chlorine atoms on
trichloroacetic acid (above left) withdraw some electron density away from the
oxygen atoms of the carboxylate (a so-called "inductive effect" –
page 147), thus helping to delocalize the negative charge. This can be seen in
the electrostatic potential surfaces in that the oxygen atoms of the
trichloroacetic acid carboxylate have less negative charge (red color) than
those of the acetic acid carboxylate (on the right). This charge delocalization
stabilizes the anion, explaining why trichloroacetic acid is about 4 orders of
magnitude more acidic than acetic acid!
Carboxylic Acids Form Tightly Associated Pairs Through Hydrogen Bonding
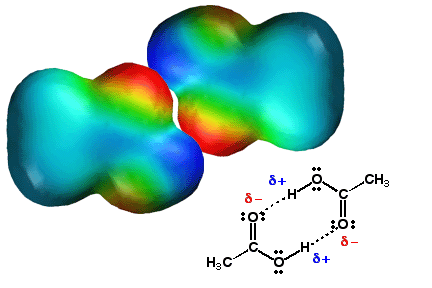
Sown above is the hydrogen
bonded dimer structure that is found for carboxylic acid molecules in a
non-polar solvent like chloroform. The hydrogen bonds are shown in yellow and
occur between the hydrogen atom of one molecule, and the carbonyl oxygen atom of
the other. Note in the electrostatic potential surface models below how this is
just partial negative charge (carbonyl oxygen atom, red color) making an
electrostatic bond with partial positive charge (hydrogen atoms, blue color).
Carboxylic acids can "stick together" via hydrogen bonding, so they
have relatively high boiling points compared to other types of molecules with
similar molecular weights.
Sources
include http://www.cm.utexas.edu/academic/courses/Spring2001/CH610B/Iverson/index.html