CH610B Sophomore Organic II
|
Dr. Brian
Pagenkopf |
|
|
|
Electrophilic Aromatic Substitution
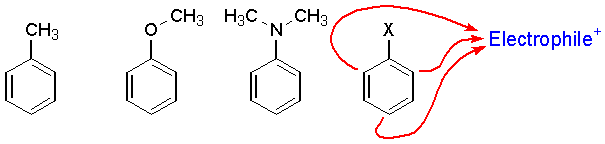
(Check out this Table). The following benzene rings are substituted with electron donating groups and activating (meaning these are more reactive than benzene). In each case, reactions with electrophiles occur at the ortho or para positions. (Why? see above resonance structures).
The following benzene rings are substituted with electron poor groups. These are deactivating (meaning the ring is less reactive than benzene). In each case, reactions with electrophiles occur at the meta position.
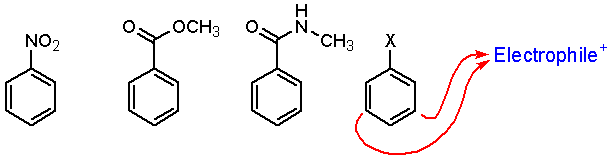
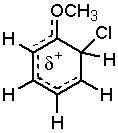
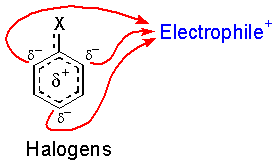
Halides are unique. The are deactivating yet direct reaction at the ortho and para positions.

The charge reactivity pattern is summarized by the following.
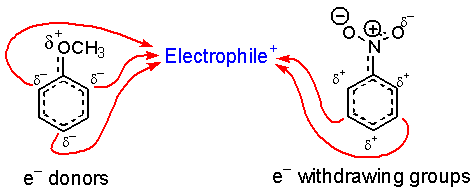
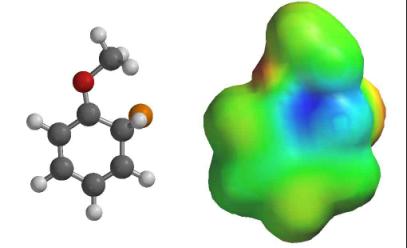
So, what’s up with the halogens? They are very electronegative and remove electron density from the ring through their sigma bond (inductive effect), but not through pi conjugation (resonance) because there are no suitable empty orbitals. A pair of electrons on the halogen is still able to donate into the ring through resonance.
Mechanism of electrophilic aromatic substitution with an activating group:
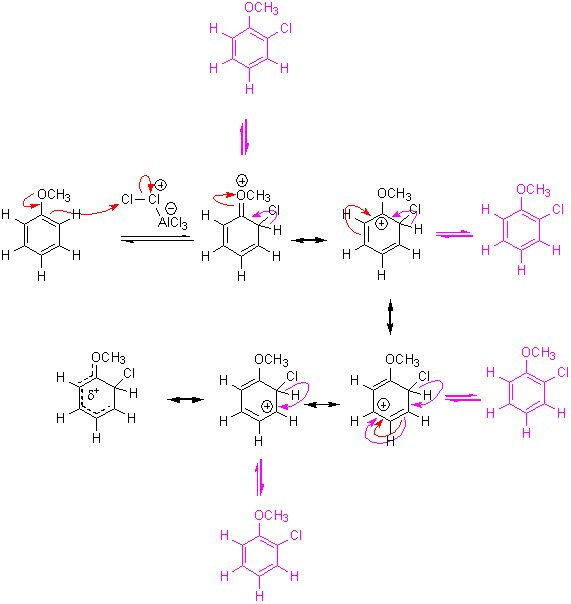
In the above mechanisms you can replace ortho attack for para attack and resulting set of resonance structures will be equivalent.
Look what happens to the charge in the ring after nucleophilic attack:
|
|
|
After the ring attacks an electrophile the aromaticity around the ring is lost! To regain aromaticity the ring merely has to lose a proton, as shown above.
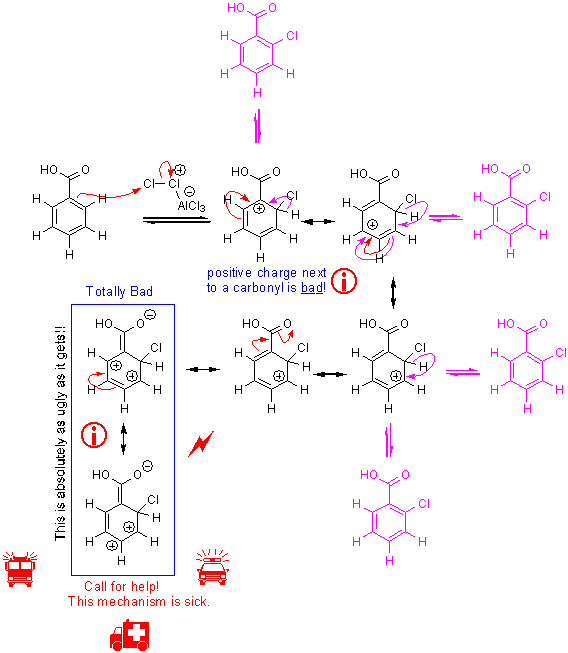
When the substituent on benzene is electron withdrawing, the mechanism looks about the same:
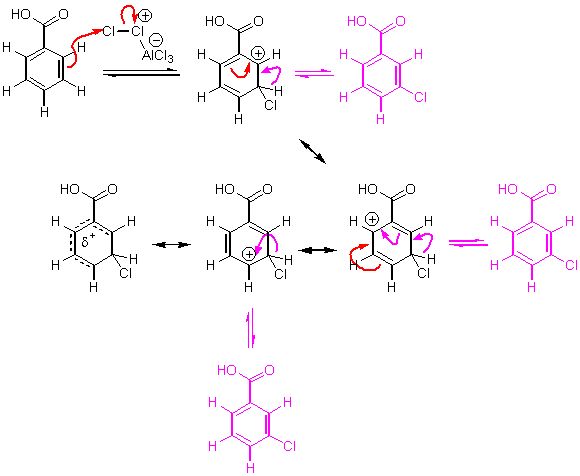
i You might wonder, “why can’t I have it attack at the ortho position anyway?” Well, it can happen, but it’s not a favorable reaction because of higher energy intermediates. We can draw a mechanism for this reaction, but look how terrible the resonance structures look of the intermediate cation. Putting an electron withdrawing group next to a positive charge is bad, just like putting two negative charges together.