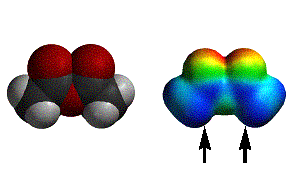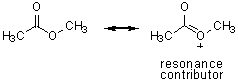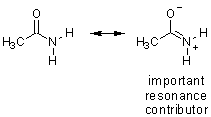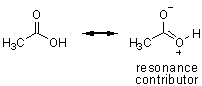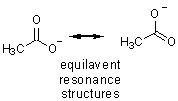|
|
|
|
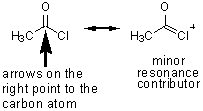
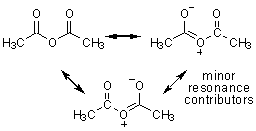
Descending down the table the carbonyl groups become less reactive as they gain better electron donors. Groups with lone pairs of electrons (such as the first two entries) may NOT be good electron donors because they are highly electronegative and excellent leaving groups in the case of chloride, or in the case of the anhydrides, stabilized by resonance (which makes them great leaving groups).
There is no hard and fast rules to carbonyl reactivity. Exceptions to this list abound, but the understanding the causes and parameters of the general trends is important.
|
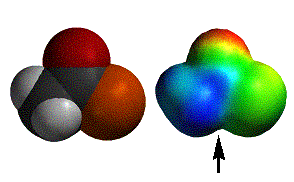
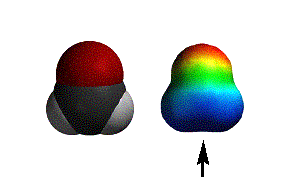
acetyl chloride |
|
|
|
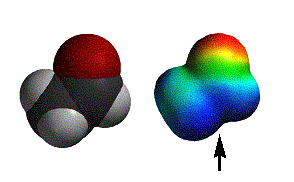
acetic anhydride |
|
|
|
formaldehyde |
|
|
|
acetaldehyde |
|
|
|
acetone |
|
|
|
|
|
|
|
|
|
|
|
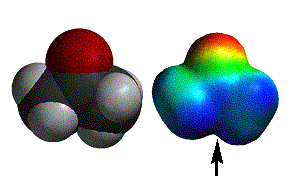
methyl acetate |
|
|
|
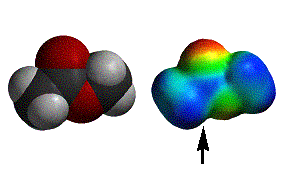
acetamide |
|
|
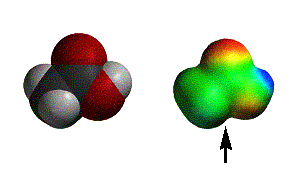
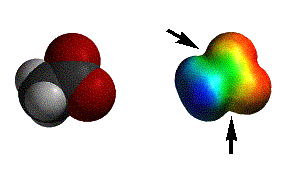
Reactions with carboxylic acids can sometimes be strange (we’ll discuss examples with amines), but generally nucleophiles deprotonate the acid to give the anion. As you can see, the anion is very electron rich and only alkyl lithium reagents add to carboxylate anions.
|
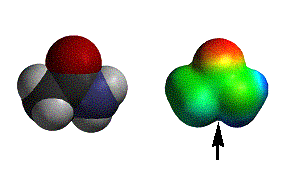
acetic acid |
|
|
|
acetic acid anion |
|
|