|
|
|
|
How to determine the oxidation state of carbon.
For a given carbon look at the groups it is attached to. Using the table below you can add up all the “charges” on that carbon and determine its oxidation state. This process is just a formalism and does not mean there’s really a charge on the carbon atom.
Group |
Bonding? |
Charge count |
|
C |
sigma |
0 |
|
C |
pi |
+1 |
|
=C |
sigma and pi |
+1/2 |
|
H |
- |
0 |
|
O |
sigma |
+1 |
|
O |
pi |
+1 |
|
=O |
sigma and pi |
+2 |
|
N |
sigma |
+1 |
|
N |
pi |
+1 |
|
=N |
sigma and pi |
+2 |
|
F, Cl, Br, I |
- |
+1 |
|
Li (organolithium) |
- |
–1 |
|
Mg (Grignard) |
- |
–1 |
|
Cu (Gilman) |
- |
–1 |
|
|
|
|
The oxidation state for several carbon atoms in the structure below is shown. Common oxidation states for carbon are –1 to + 4. An oxidation state of 1/2 for a double bond makes no sense, but it does if you consider both carbons of the double bond.
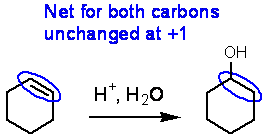
Here’s a molecule that shows various oxidation states:

Remember this: You can NEVER effect an oxidation or reduction of an organic compound with just water! Consider the hydration of an alkene and see what happens to the oxidation states of the two carbons.
Determining the oxidation state of carbon can be helpful in figuring out what reagent you need to effect a particular reaction. In the following reaction diagram involving carbonyl chemistry, you’ll see monitoring the oxidation state can help you determine what kind of reagent is needed. We start with 1-propanol with the indicated carbon atom at a +1 oxidation state. a) must be an oxidation (PCC); b) oxidation (H2SO4, K2CrO4); c) no redox (reduction/oxidation) happened in this step (H+, EtOH); d) no redox (H+, MeOH); e) amide formation, no redox (HNMe2); f) reduction from +3 to +1 (LiAlH4); g) reduction (DIBAL-H); h) this doesn’t look like a redox reaction, but note that while the electrophilic carbon goes from +2 to +1 (reduction) that in the Grignard the carbon bonded to Mg has gone from 0 to +1. So, the overall reaction is redox neutral (as all reactions must be). i) enamine formation, no redox ( H+, tetrahydropyrrole); j) acetal formation, no redox (H+, ethylene glycol).
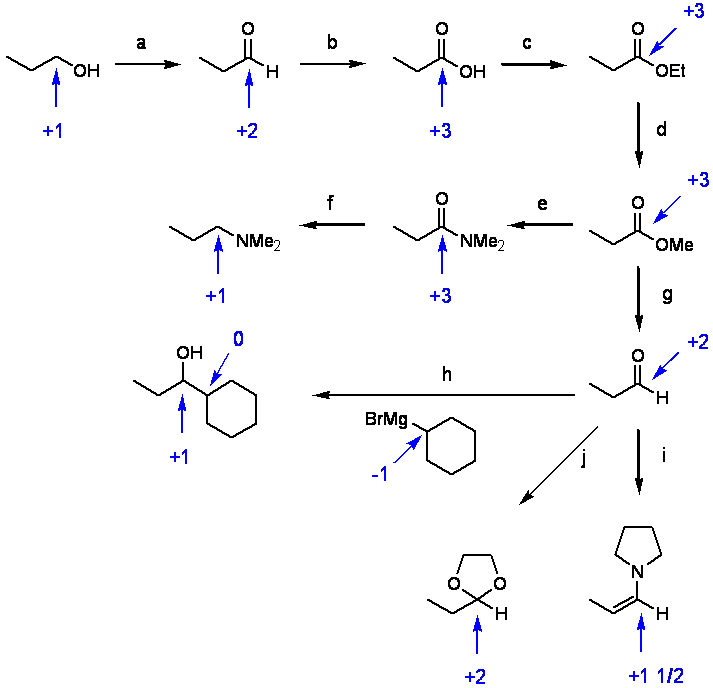
Disclaimer: this simplified model works well for monitoring what’s happening with an individual functional group, but the oxidation state determination does not apply globally to the molecule. The easiest modification that makes this a more general model is to view C-C pi bonds as worth +1/2 and not as +1. See i above.