CH610B Sophomore Organic II
|
Dr. Brian
Pagenkopf |
|
|
|
Hydrides, Reducing agents, and Sources of Nucleophilic H:¯
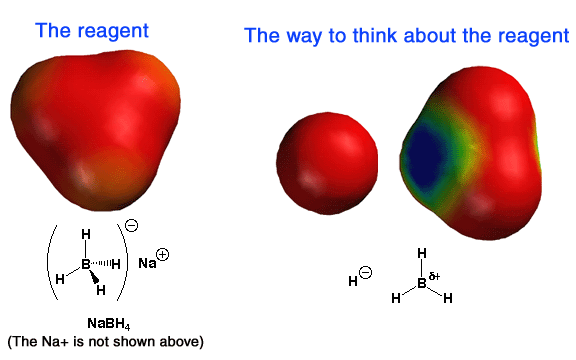
Above are electrostatic
potential surfaces that explain a great deal about the reactivity of NaBH4.
The best way to think about NaBH4 is as an H:¯ atom (a so-called hydride ion) bonded to the Lewis
acid BH3, as indicated above on the right. This way of thinking
helps explain why the NaBH4 reagent reacts by donating a
nucleophilic H:¯ atom to
electrophiles such as the carbon atom of carbonyls. On the left is shown the
actual structure of BH4¯;
notice the overall negative charge (red color). On the right for comparison is
BH3, showing a partial positive charge (blue color) on the boron
atom and a partial negative charge (red color) on the hydrogen atoms,
consistent with the electronegativies of those types of atoms. The partial
positive charge on boron explains its Lewis acid (electron pair seeking)
properties, i.e. why it forms such a nice complex with the
negatively-charged oxygen atom of the tetrahedral intermediate.
However, a surprising point
to remember is that BH4¯ does
not behave chemically much like BH3 at all!
For example, BH4¯ does
not readily react with double bonds (hydroboration).
Sources
include http://www.cm.utexas.edu/academic/courses/Spring2001/CH610B/Iverson/index.html