CH610B Sophomore Organic II
|
Dr. Brian
Pagenkopf |
|
|
|
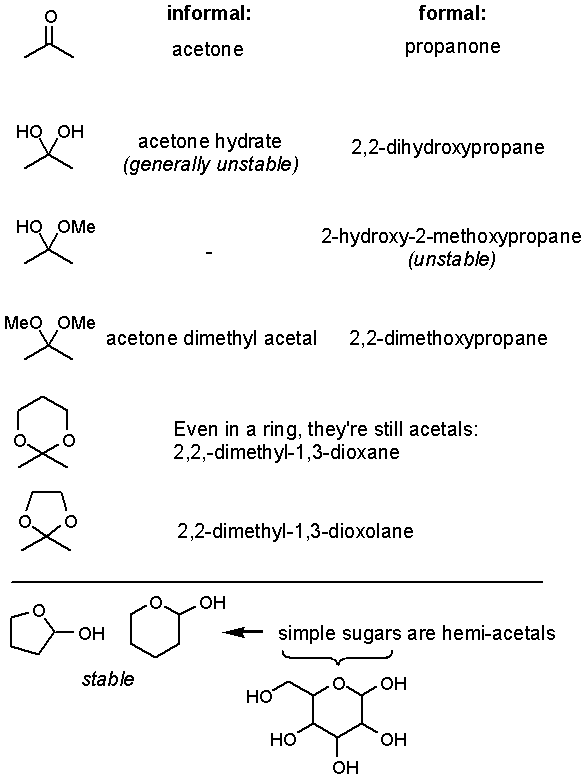
Think of ďacetalĒ as simply another functional group.† Write it into the front cover of your text book.† An acetal is a molecule that contains a carbon bound to two OR or OAr groups (R not = H).† A hemi-acetal (hemi = half) is a molecule that contains a carbon bound to an alcohol (OH) and an OR or OAr.†† In general hemi-acetals are unstable except when they form a ring.† Sometimes youíll see acetals made from ketones called ketals.
The most important rule in acid or base catalyzed carbonyl additions: proton transfers are rapid, facile, easy, quick, fast, ubiquitous and central to catalysis in protic media (solvents like alcohols).† This means that when writing a mechanism for acid catalyzed (or base catalyzed) carbonyl chemistry you may move protons back and forth from oxygens or nitrogens in substrates, reagents or solvents as often as you like and it wont be too much.† This answers the question, ďBut where did that proton come from?Ē Donít worry about it, thereís more than enough protons around in alcohol or water solvents regardless of their pH.
Another useful generality: addition reactions carried out under acid or base catalysis are reversible.
Q: Sugars are acetals?
A: Yes, they just look a little more intimidating because there are so many diastereomers (shown as Fisher projections). What does this say about the stability of cyclic acetals of various ring sizes?
Because sugars are so important, the stereochemical conformation at the acetal carbon has a special name: the anomeric carbon.† Also, the OH at the anomeric center is designated beta when the alcohol is up and alpha when the OH is down.† This is relative to the terminal CH≠2OH group.† The alpha and beta forms are in equilibrium, and behave like other acetals: they react with MeOH/H+, can be oxidized and reduced.
Q: How does acid catalysis work with amines?† Arenít the nitrogens protonated and therefore not nucleophilic?†
A: The protonation of an amine is reversible.† While the bulk of the acid catalyst will be associated with the amines, some of the free acid will be available to catalyze the carbonyl addition reaction.
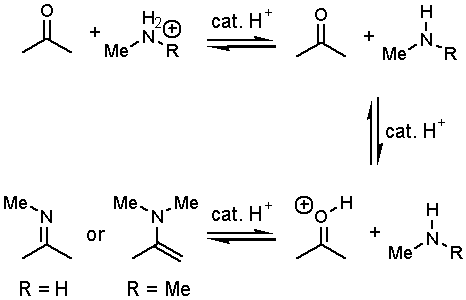
Goal: Explain why primary and secondary amines (see the above reaction) give different products when they react with aldehydes or ketones.
Rules specific to base catalyzed reactions in protic solvents (like alcohols): acidic and basic conditions donít exist at the same time.† Keeping this simple idea in mind, it doesnít make sense to propose mechanism in which a carbonyl is activated towards nucleophilic attack through protonation (by an acidic proton as we did here) when the media is basic. Another way of saying this: donít propose using strong acids when all thatís available are bases.† However, proton transfers are still really fast regardless of pH.
This is illustrated well in the Wolff-Kishner Reduction.† What steps of the Wolff-Kishner reduction are reversible and which arenít?