CH610B Sophomore Organic II
|
Dr. Brian
Pagenkopf |
|
|
|
All
nucleophiles add to aldehydes the same way!
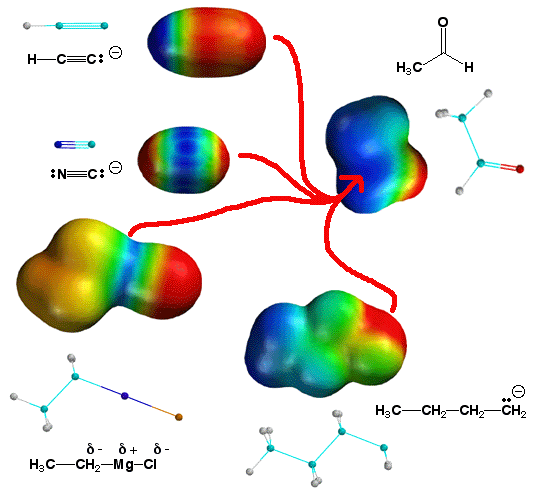
Four addition reactions to
aldehydes and ketones. In each case, the nucleophilic site of the molecule
(electron rich, red or orange color) interacts with the carbonyl carbon atom
that has a partial positive charge (blue color). Note that in the case of CN-
and the Grignard reaction it is not so obvious which nucleophilic site reacts
with the carbonyl. Each has more than one partial negative charge. The site
that reacts is not always the one with the most electron density, it is the one
that can form the most stable bond. Reactions need a motive to react, namely
stable products (lower in energy than starting materials) as well as the
opportunity (nucleophile reacts with electrophile). In the case of the Grignard
and CN- reaction, the more stable bonds by far are the carbon-carbon bonds, so
these are what form in the reactions.
Sources
http://www.cm.utexas.edu/academic/courses/Spring2001/CH610B/Iverson/index.html