CH610B Sophomore Organic II
|
Dr. Brian
Pagenkopf |
|
|
|
Compare the molecular orbitals and electrostatic potential maps of ethylene and formaldehyde. They share many similarities, but note the relative sizes of the anti bonding orbitals and distribution of electron density. (ethylene on the left, formaldehyde on the right).
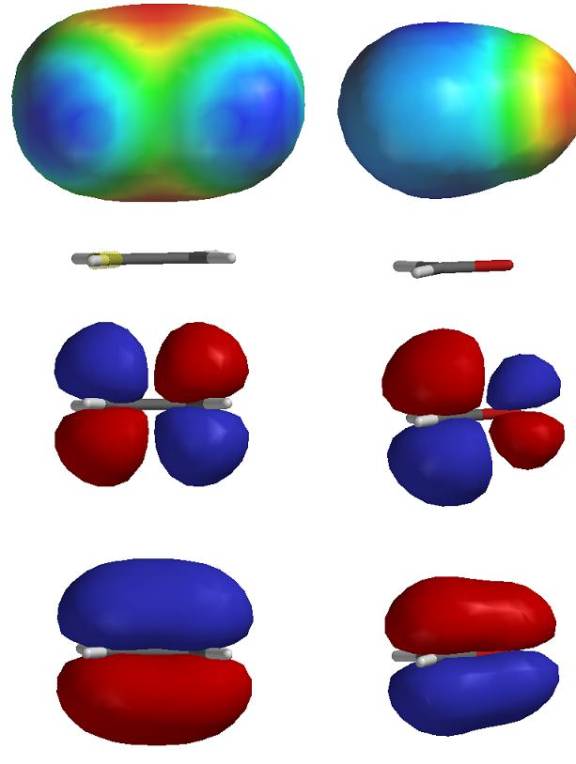
Nucleophiles must add their electrons into an empty (anti bonding) orbital.
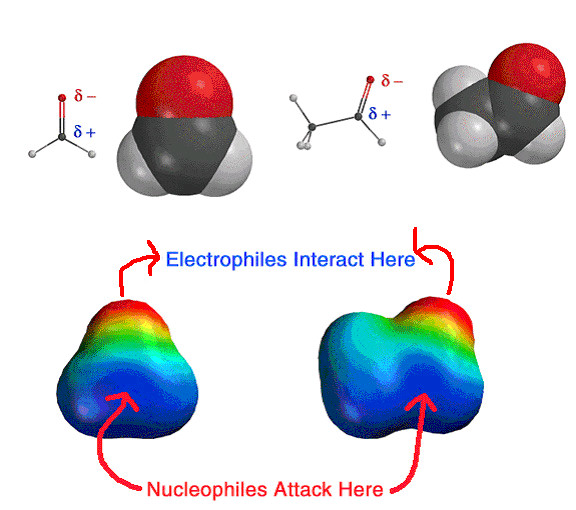
Shown above is the
electrostatic potential surface of a carbonyl, in this case formaldehyde (left)
and acetaldehyde (right). This picture is most of what you need to remember
about carbonyls in terms of nucleophilic attack. The electrostatic force will
direct nucleophiles to the carbonyl carbon atom, while electrophiles (Lewis
acids and protons) will be directed to the oxygen atom. The pi bond will break
upon nucleophilic attack to create the TETRAHEDRAL INTERMEDIATE.
Sources
include
http://www.cm.utexas.edu/academic/courses/Spring2001/CH610B/Iverson/index.html