CH610B Sophomore Organic II
|
Dr. Brian
Pagenkopf |
|
|
|
Enolates: Stabilized by
resonance
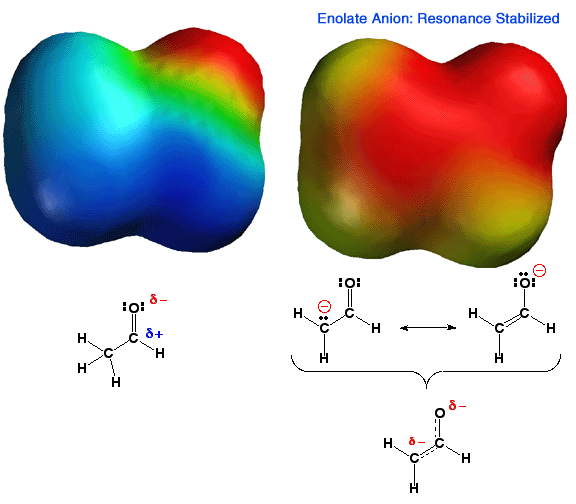
Shown above is the electrostatic potential energy surface for an enolate ion. Note how the negative charge, (red color) is distributed over both the alpha carbon atom and the oxygen atom of the enolate. You should be able to draw resonance forms that describe this charge distribution. This delocalization of charge makes this anion more stable than an anion with a localized charge. Thus, carbonyl groups that have an alpha proton are MUCH more acidic than other types of C-H bonds such as alkanes or alkenes. In addition, enolates are very reactive nucleophiles, being able to react at either the alpha carbon atom or the oxygen atom. That is, they are not only highly reactive, they are also "schizophrenic" nucleophiles. Because carbon-oxygen double bonds are stronger than carbon-carbon double bonds, as well as other factors like solvation etc., reactions with electrophiles, especially carbon electrophiles, occur primarily at the carbon atom of the enolate.
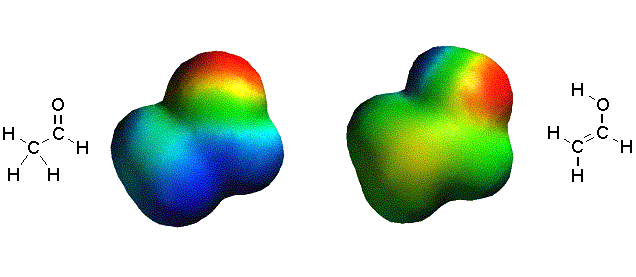
Below
you can see that in a neutral tautomer of acetaldehyde there’s much less
electron density on the carbon next to the carbonyl compared to the anionic
enolate above.
Sources
include
http://www.cm.utexas.edu/academic/courses/Spring2001/CH610B/Iverson/index.html