|
|
|
|
Hereís an example with an aldehyde.
Addition of a nucleophile to a carbonyl will give a tetrahedral intermediate, and predicting what happens next is the same as predicting the carbonylís chemistry.† The reaction may reverse, stop there, or collapse to a new carbonyl compound (which may or may not go on to react again).
Addition to an aldehyde gives a tetrahedral intermediate:
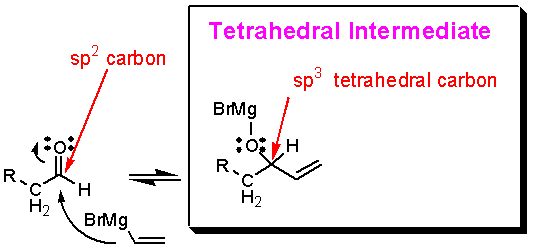
What happens to the tetrahedral intermediate next?† In the general scheme there were four possible outcomes.† With aldehydes thereís only one pathway:
The reaction stops after addition of one nucleophile:

Grignard addition to aldehydes always gives secondary alcohols.†
Grignard addition to ketones always gives tertiary alcohols.