CH610B Sophomore Organic II
|
Dr. Brian Pagenkopf |
|
|
|
Amide and Ester Structure
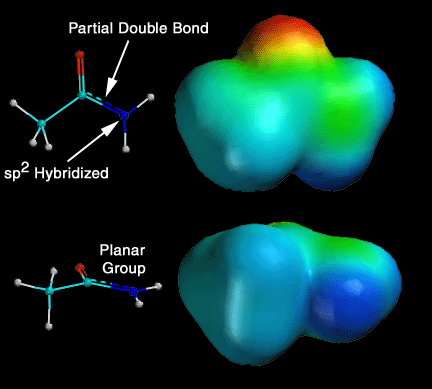
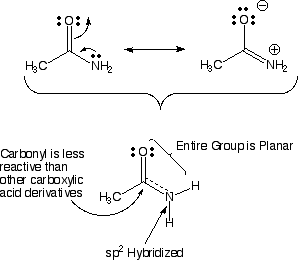
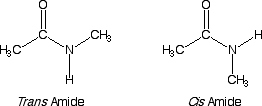
Amide and ester structure is different than you might think!! It turns out that the lone pair of electrons on the amide nitrogen atom takes part in resonance so that you should think of amides as the hybrid of the two resonance contributing structures shown above. This happens because pi electrons prefer to delocalize, and this resonance amounts to pi electron delocalization. This delocalization has several important consequences for amide groups. First, there is a partial double bond between the carbonyl carbon atom and the nitrogen atom of the amide. Second, the nitrogen atom is sp2 hybridized, because otherwise you could not have the 2p orbital to take part in the pi electron delocalization. Third, the entire amide group is planar. Fourth, the carbonyl carbon atom is much less reactive as an electrophile compared to other carboxylic acid derivatives. Fifth, since the C-N bond of an amide cannot rotate freely (partial double bond) you can have "cis" and "trans" amides. The question can be asked, why would the amide nitrogen become sp2 hybridized, when by the VSEPR model, it should be sp3 hybridized (4 areas of electron density around it)? The answer is that it costs a little energy to go from sp3 to sp2 hybridized, but because pi electron delocalization provides stabilization, overall the molecule is lower in energy with an sp2 hybridized amide nitrogen that allows pi delocalization (the energy gained by pi delocalization outweighs the cost of sp2 hybridizaiton here).
Esters are like amides, but to a lesser extent. Thus, esters
tend to be planar and the corresponding ester oxygen atom is somewhere between
sp3 and sp2 hybridized.