CH610B Sophomore Organic II
|
Dr. Brian
Pagenkopf |
|
|
|
The Wittig Reaction: |
|
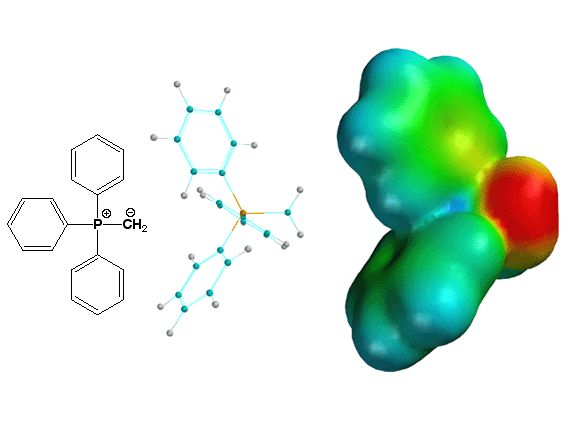
Above is shown the simplest
Wittig reagent, namely the ylide made from a methyl halide and triphenyl phosphine.
Note how in the electrostatic potential surface the negative charge is on the
carbon atom, explaining why it is such a good nucleophile and reacts with
carbonyl groups.
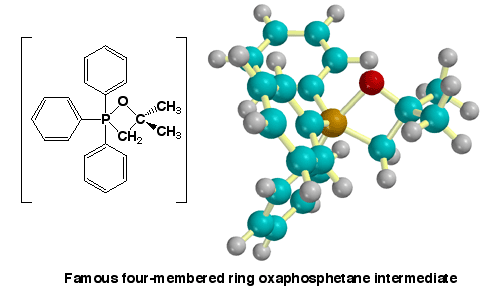
Shown here is the
four-membered ring intermediate that occurs when the Wittig reagent derived
from a methyl halide reacts with acetone. The unique feature of the Wittig reaction
is the mechanism in which the famous four-membered ring intermediate, called an
oxaphophetane, is formed.
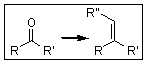
The Wittig Reaction is great
for converting aldehydes and ketones into alkenes! Although there are two competing
models on the correct mechanism for this reaction, we can employ what we
already know to explain it.
Sources
include
http://www.cm.utexas.edu/academic/courses/Spring2001/CH610B/Iverson/index.html