Click here to return to course outline.
Click here to return to W.R. Church's home page.
Click here to return to Earth Science course list.
A mineral is defined as a naturally
occurring element or compound having an unique internal ordered
structure and a definite chemical composition.
Some minerals, e.g. halite and galena, may have the same internal structure
but different chemical compositions, whereas other minerals, e.g. kyanite
and sillimanite or calcite and aragonite, may have the same chemical composition
but a different internal structure. The latter are known as polymorphs.
All minerals can be organised into
about 30 mineral groups, composed of varying combinations of the 81 elements
that exist in nature ( that is, excluding Tc and Pm, which do not exist
in nature, and the unstable heavy radioactive elements).
Mineral Classification
The following is a standard mineral classification based on the chemical composition of the anionic component of the mineral, with the silicates further divided into classes according to the arrangement of the silica tetrahedra forming the silicate mineral. (Minerals and formulae that need to be known are underlined.)
Web
link to 'webmineral' (http://webmineral.com/)
Web
link to 'Athena' (http://un2sg4.unige.ch/athena/mineral/mineral.html)
Silicates
Nesosilicates (Ortho)
Cyclosilicates - beryl, cordierite [(Mg,Fe)2Al4Si5O18.nH2O],
- tourmaline [(Na, Li, Ca, Mg Al) Fe6B3O9.Si6O18(OH)4]
Inosilicates (Chain)
- clino-pyroxenes [Ca(Mg,Fe)Si2O6]; ortho-pyroxenes [(Mg,Fe)2Si2O6]; jadeite [NaAlSi2O6]
- amphiboles [Ca2(Fe,Mg)5 Si8O22(OH)2-Ca2(Fe,Mg)4Al2 Si7O22(OH)2]
- pyroxenoides, - pectolite, rhodonite [MnSiO3], wollastonite [CaSiO3]
Phyllosilicates (Sheet)
- serpentine [Mg3 Si2O5(OH)4], talc [(Fe,Mg)3 Si4O10(OH)2]
- chlorite [(Fe,Mg)2Al2 SiO5(OH)4- Fe,Mg)2Al2Si3O10(OH)2]
- micas - muscovite [KAl3 Si3O10(OH)2], biotite [KAl(Fe,Mg)3Si3O10(OH)2
- clays - kaolinite [Al2 Si2O5(OH)4 (2-layer clay)], pyrophyllite [Al2Si4O10(OH)2 (3-layer clay)]
-smectite (Mg.5,1.75Al)(Si3.75,Al.25)O10(OH)2 (3 layer)
-illite K.5Al2 Al.5Si3.5O10(OH)2
Tectosilicates (Framework)
- silica - quartz [SiO2], chalcedony, coesite
- feldspars - plagioclase [CaAl2Si2O8 (= anorthite)-NaAlSi3O8 (= albite)], orthoclase, microcline [KAlSi3O8]
- feldspathoids - nepheline [NaAlSiO4], leucite [KAlSi2O6]
- zeolites - analcime [NaAlSi2O6.H2O]
ORE MINERALS
| Metallic | - Native - gold [Au], silver [Ag], copper [Cu], iron [Fe], platinum [Pt] |
| Non-Metallic | - Native - sulphur [S], graphite [C], diamond [C] |
| Sulphides | - troilite (meteorites) [FeS], pyrite [FeS2], pyrrhotite [Fe(1-x)S], marcasite [FeS2], |
| Arsenides | - pentlandite [(Fe,Ni)9S8], galena [PbS], sphalerite [ZnS], chalcopyrite [CuFeS2], |
| Antimonides | - bornite [Cu5FeS4],
covellite [CuS], chalcocite [Cu2S],
arsenopyrite [FeAsS], realgar [AsS],
stibnite [Sb2S3], molybdenite [MoS2],
cobaltite [(Co,Fe)AsS],
cinnabar [HgS], argentite [Ag2S] |
| Sulphosalts | - tetrahedrite-tennantite [Cu12(Sb,As)4S13 + c.100 other rare mineral species of the form [(C,Ag,Pb)x(As,Sb,Bi)ySz] |
| Tellurides | - calaverite [AuTe2]; sylvanite [Au,AgTe2] |
INDUSTRIAL
MINERALS
| Arsenates | - adamite [Zn2AsO4(OH)], annabergite[Ni8(AsO4)2.8H2O] |
| Borates | - borax [Na2B4O7.10H2O] |
| Carbonates | - calcite [CaCO3],
magnesite [MgCO3], siderite [FeCO3],
dolomite [CaMg(CO3)2],
rhodocrosite [MnCO3], strontianite [SrCO3], malachite [Cu2CO3(OH)2], azurite [Cu3(CO3)2.(OH)2] |
| Chromates | - crocoite [PbCrO4] |
| Halides | - halite [NaCl], sylvite [KCl], fluorite [CaF2] |
| Hydroxides | - goethite [Fe2O3.H2O],
brucite [Mg(OH)2], manganite [Mn2O3.H2O]
uraninite [UO2], gibbsite Al(OH)3], diaspore [Al2O3.H2O] |
| Iodates | - lautarite [Ca(IO3)2 |
| Molybdates | - wulfenite[PbMoO4] |
| Nitrates | - soda-nitre [NaNO3], nitre [KNO3] |
| Oxides | - spinel [(Mg,Fe)(Al,Cr,Fe3)2O4],
magnetite, chromite;
- ilmenite [FeTiO3 (FeO.TiO2)] - hematite [Fe2O3],corundum [Al2O3], - cassiterite [SnO2], - pyrolusite [MnO2], rutile [TiO2] |
| Phosphates | - apatite [Ca5(PO4)3.Cl,F], turquoise [CuAl6(PO4)4(OH)8.4H2O], monazite [Ce,La,Y,Th)PO4] |
| Sulphates | - anhydrite [CaSO4], gypsum [CaSO4.2H2O], barite [BaSO4] |
| Tungstates | - scheelite [CaWO4], wolframite [(Fe,Mn)WO4] |
| Vanadates | - carnotite [K2(UO2)2(VO4)2.3H2O] |
The identity of most common minerals can be guessed by a simple examination of the mineral's crystal form (if such can be observed); habit (e.g. bladed, acicular, tabular); colour; cleavage and fracture; hardness; lustre; streak; density (specific gravity); magnetism and taste. A definitive and exact identity however requires the use of an X-RAY diffractometer to establish the structure of the mineral, and a MICROPROBE analyzer to determine its chemical composition. The latter is important in some branches of geology because only by comparing minerals as chemical entities can their equivalence be established. To test, for example, the proposition that a rock composed of albite has the same chemical composition as one composed of jadeite and quartz, or that a rock composed of anorthite and orthopyroxene is chemically equivalent to a rock composed of garnet and quartz, it is necessary to know the chemical compositions of the minerals concerned.
The Structure of Silicate minerals
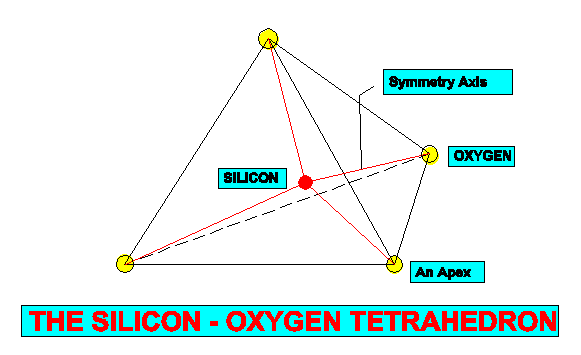
All silicates are formed of various arrangements of anionic silica-oxygen tetrahdra (SiO4), each tetrahedron being composed of a single atom of silica at its centre and an oxygen atom at each of its four apices.
In the case of olivine, the tetrahedra are arranged in sets of parallel lines, such that the tetrahedra in adjacent arrays point in opposite directions, and the adjacent tetrahedra in the same array alternatively point 'up' and 'down'.
Crystallographic structure of Olivine
More formally, as
will be explained in class with the aid of a 3-dimensional model,
the tetrahedra are arranged according to the following rules:
1) when viewed in the direction
of the (a) axis (i.e. the direction normal to the 100 plane, see Orthogonal
Crystallographic Axes) the tetrahedra form linear arrays parallel
to the (b) axis such that one face of each tetrahedron is parallel to 100
and the apical axis (the symmetry axis connecting the centre of the tetrahedron
to its one of its apices) normal to this plane is parallel to (a);
2) adjacent tetrahedra within each
array face in opposite directions;
3) adjacent tetrahedral arrays
display opposing polarity;
4) all oxygens lie in sheets parallel
to the 100 and 001 plane;
5) Mg cations distributed between
the tetrahedral sets are each surrounded by 6 oxygens (coordination = 6).
The total negative charge of the four divalent oxygens of each tetrahedron is 8 charge units. Since each Si ion within the tetrahedron has only four positive charge units, the charge needed to balance the remaining negative charge of four units of the surrounding oxygen atoms must be provided by the charge of two positive divalent atoms of Mg or Fe. [NOTE, as a matter of interest to those who will be taking ES205: since each divalent Mg/Fe atom is surrounded by 6 atoms of oxygen, each Mg/Fe atom is capable of contributing 1/3 (= 2 charge units/6 oxygens) of a charge to the charge balance. Consequently, each of the four oxygen atoms of the silica tetrahedron must be bonded to (surrounded by) 3 atoms of Mg/Fe, such that, numerically, 4 oxygens x 3 Mg/Fe x 1/3 charge = 4 charge units.]
In the case of pyroxene minerals (Inosilicates) the tetrahedra are linked to form a chain where two oxygens of each tetrahedral unit are shared by adjacent tetrahedra. The shared oxygens all lie in the same line. Adjacent tetrahedra in the chain face in opposite directions when viewed 'downwards' in one direction normal to the line of shared oxygens, but all face upwards in the same direction when viewed 'from the side' in the other direction normal to line of shared oxygens. In this case the number of oxygen atoms surrounding the two Si atoms (8 positive charges) of adjacent tetrahedra equals 6 ( = 12 negative charges) rather than 8 as in the case of adjacent olivine tetrahedra. The charge deficiency of 4 charge units is made up by 1 atom of Ca and one of Mg or Fe in the case of clinopyroxene, or 2 atoms of Mg or Fe in the case of orthopyroxene.
Crystallographic structureof Pyroxene
Amphiboles (Inosilicates) are formed of a tetrahedral double chain, such that the anion complex is Si8O22(OH)214- with the charge imbalance of 14 cancelled by some linked combination of Ca, Na, K, Mg, Fe, Ti, or Al, whereas micas [Phyllosilicates - KAl3 Si3O10(OH)2] are formed of tetrahedra arranged in parallel sheets.
In feldspars (Tectosilicates), the
negative charge of the Si3O8----
tetrahedral arrangement in albite is balanced by the presence of two cation
species, a trivalent cation of Al and a univalent cation of Na.
The chemical composition of silicate minerals
Most minerals are composed of a relatively small number of what are known as major elements, usually shown ordered in the following manner to reflect the valency of the elements:
SiO2 TiO2 (= 4) Al2O3 Fe2O3 (= 3) FeO MnO MgO CaO (= 2) Na2O K2O (= 1) P2O5 (= 5) LOI (=H2O + CO2),
where SiO2, Al2O3, and sometimes TiO2, commonly form the anionic radical in minerals. Some minerals such as olivine may contain only one major element (Mg or Fe) as a cationic component, whereas other minerals such as amphibole (the garbage mineral!) may contain Ca, Na, K, Mg, Fe, Al, Mn, Ti, and (OH).
Learning the chemical formulae of common minerals is made easier by first learning the formula of the anionic root, e.g. olivines are SiO4, pyroxenes, Si2O6, feldspars Si3O8(AlSi2O8), amphiboles Si8O22(OH) 2, etc. It is then necessary to learn that only Mg and Fe can be incorporated in olivine (a mafic mineral), that Ca, K, and Na are the only elements that can be present in feldspar (a felsic mineral), etc. Regrettably there is no easy way to predict the cationic component of each mineral group. Note also that the felspathoidal minerals nepheline (NaAlSiO4) and leucite (KAlSi2O6). although having the same anionic root as olivine and pyroxene, respectively, do not belong to these groups! This information can only be learned by rote. SORRY!!
End members and Solid Solutions
Olivine is a nesosilicate composed of isolated SiO4 tetrahedra linked by atoms of Mg or Fe. If all the linking atoms are Mg, the olivine is known as Forsterite and has the composition Mg2SiO4; if all the linking atoms are Fe, the olivine is known as Fayalite and has the composition Fe2SiO4. Forsterite and Fayalite are known as 'end members' of olivine. The end member compositions are capable of completely dissolving one in the other, and the proportion of Mg and Fe in olivine is therefore only limited by the composition of the rock material. Olivine is said to be a solid solution of the end members forsterite and fayalite. The formulae of minerals representing solid solutions are usually written in the form:
(X,Y)ASiBOC
for example, (Mg,Fe)2SiO4
in the case of olivine, or Ca(Mg,Fe)Si2O6
in the case of clinopyroxene, or (Mg,Fe) (Al,Cr,Fe3+)O4
in the case of spinel, etc.
Minerals may also change composition by undergoing a coupled substitution. For example actinolite/tremolite changes to hornblende by a coupled substitution of Si by Al and of Fe/Mg by Al, and serpentine can convert to chlorite by a coupled substitution of Si by Al and of Mg by Al. In both cases note that the charge balance is preserved by the element substitution, i.e. 4 (Si)+ 2 (Mg/Fe) = 3 (Al) + 3 (Al). Such changes reflect increasing temperature of crystallization.
Trace elements
Elements that substitute for major elements in minerals are known as trace elements. In the case of igneous minerals, elements that prefer to concentrate in solid mineral phases rather than in the melt (magma) from which the minerals are crystallizing are called compatible trace elements. Examples include nickel in olivine, rubidium in K-feldspar, strontium in plagioclase and calcite, yttrium in garnet, titanium and vanadium in magnetite, and scandium in pyroxene.
Mineral Properties
The minerals constituting 'Mohs Scale of Relative Hardness' are:
10 - diamond (cuts glass),
9 - corundum, 8 - topaz, 7 - quartz (scratches glass easily), 6 - orthoclase
(scratches glass with difficulty),
5 - apatite (= iron nail or
knife), 4 - fluorite, 3 - calcite, 2.5 - fingernail, 2 - gypsum, 1 - talc
and the major mineral and rock forming elements are
SiO2 TiO2 Al2O3 Fe2O3 FeO MnO MgO CaO Na2O K2O P2O5 LOI (= H2O + CO2)
The common rock forming minerals and their diagnostic features (colour, crystal form, cleavage, fracture, hardness, luster (e.g. vitreous, resinous, metallic, satin), density, streak, reaction to acid, etc) are:
Olivine, Forsterite - Fayalite (Mg,Fe)2SiO4
Green, vitreous, no cleavage, hardness 6.5, D - 3.5
Clinopyroxene, Diopside - Hedenbergite Ca(Mg,Fe)Si2O6
Black (augite, igneous) to green (diopside, metamorphic), vitreous, monoclinic, prismatic, square-like cross sections, 90 degree cleavage, hardness 6, D - 3.2
Orthopyroxene, Enstatite - Ferrosilite) (Mg,Fe)2Si2O6
Bronze coloured, vitreous, orthorhombic, prismatic, hardness 6, D - 3.2
Sodium pyroxene, Jadeite, NaAlSi2O6 (no sample, a precious stone)
Jade green! monoclinic, hardness 6, D - 3.4,
Plagioclase, Anorthite - Albite CaAl2Si2O8 - NaAlSi3O8
White, vitreous, platy, tetragonal, two cleavages at nearly right angles, twinned with twins apparent as parallel striations on cleavage surfaces, zoned, hardness 6, D -2.6-2.7 (K-feldspar does not show twinning striations)
Ilmenite, FeTiO3 (no sample)
Black metallic lustre, slightly magnetic, hardness 6, D - 4-5
Magnetite, Fe3O4
Similar to ilmenite but strongly magnetic, octahedral crystals, D - 5.2
Spinel, MgAl2O4 (with bronzite sample)
Black, vitreous, octohedral, hardness 8, D- 4.
Pyrite, FeS2
Brassy yellow(fools gold), striated cubes, reacts with acid to form H2S, hardness 6, D - 5
Pyrrhotite, Fe7S8-FeS
Bronze-yellow (a whiter yellow than pyrite); magnetic, hardness 4, D - 4.6
Pentlandite, (Fe,Ni)9S8 (NiS)
Dark bronze , hardness 3.5-4; D - 4.6-5; major nickel ore at Sudbury; invariably associated with pyrrhotite therefore giving the appearance of being magnetic
Orthoclase, KAlSi3O8
White, pink, or brown, vitreous, 2 cleavage directions at 90 degrees, hardness 6, D -2.6.
Muscovite, KAl3Si3O10 (OH)2
White-grey, pseudo-hexagonal six sided (perfect cleavage, reflective, hardness 2.5, D - 2.8
Biotite, KAl(Fe,Mg)3Si3O10(OH)2
Brown, otherwise like muscovite
Quartz, SiO2
Clear to milky, can be rose, hexagonal prisms with rhombohedral faces, no cleavage, conchoidal fracture, hardness 7, D - 2.7
Apatite, Ca5(PO4)3.F,Cl
Hexagonal, prismatic, light green, vitreous, poor cleavage, hardness 5, D - 3.2.
Tourmaline, (contains Boron)
Hexagonal prismatic crystals with a rounded triangular X-section, striated sides, hardness 7.5, D - 3.1
Fluorite CaF2
Commonly purple/violet/mauve/blue, cubic, vitreous crystals associated with late-stage quartz-feldspar (+apatite) granites and syenites, but also as calcite-fluorite rocks, hardness 4, D - 3.18.
Amphibole, Tremolite-Actinolite Ca2(Mg,Fe)5Si8O22(OH)2
Colourless to grey (tremolite), dark green to black (actinolite), vitreous, acicular, hardness 5-6; D - 3 - 3.4
Hornblende, - Ca2(Mg,Fe)4Al2Si7O22(OH)2
Black, monoclinic, prismatic, vitreous, radiating acicular, 2 cleavages at 120 degrees, hardness 5.5-6, D - 3.2
Glaucophane, Na2Mg3Al2Si8O22(OH)2
Blue to purple, vitreous, cross cleavage at 120 degrees, acicular, hardness 6, D - 3.3
Garnet, - grossularite (Ca), pyrope (Mg), almandine (Fe), spessartine (Mn), (Ca,Mg,Fe, Mn)3Al2Si3O12
Red to pink, green, brown, vitreous, commonly euhedral,
dodecahedral, no cleavage but may be fractured, hardness 7, D - 3.5-4.0
Spessartine is a low temperature form of garnet, and
is commonly purple in colour.
Epidote, Ca2Al3Si3O12(OH)
Pistachio Apple green (but zoisite is pink), one perfect cleavage, vitreous, hardness 6, D - 3.2
Chlorite, (Mg,Fe)2Al2SiO5(OH)4 - (Mg,Fe)2Al2Si3O10(OH)2
Black to green, vitreous luster, well cleaved like micas, hardness 2, D - 3.0
Kyanite, Al2SiO5
Commonly blue but sometimes white, long tabular or bladed crystals, cleavage parallel to length of crystal, fractures across length of crystal; hardness 6, D - 3.5
Corundum, Al2O3
Rubies and Sapphires, hexagonal barrel shaped, no cleavage, hardness 9, D - 4.
Talc, Mg3Si4010(OH)2
White, fibrous, very easily scratched; hardness 1, D - 2.58-2.83
Serpentine, Mg3Si2O5(OH)4
Commonly green sometimes white, commonly resinous; veined by asbestos, hardness 2.5-3.5, D - 2.55-2.6
Pyrophyllite, Al2Si4010(OH)2
White, pearly lustre, hardness 1-2, D - 2.7-2.9
Kaolinite, Al2Si2O5(OH)4
White, clay, hardness 2-2.5, D - 2.61-2.68
Calcite, CaCO3
Clear to white, vitreous luster, rhombohedrons, cleaved in three directions, hardness 3, reacts to acid, hardness 3, D - 3
Dolomite, CaMg(CO3)2
Light pink rhomb crystals, weathers yellow-orange, effervesces with acid if powdered, hardness 4, D - 3
Siderite, FeCO3 (no sample)
Brown vitreous rhombs, curved faces, hardness 3.5, D - 4
Gypsum, CaSO4.2H2O (-> anhydrite CaSO4)
Transparent (Selenite), fibrous (Satin spar), fine grained (Alabaster), hardness 2, D - 2.2 (Anhydrite 3)
Barite, BaSO4
Tabular, vitreous, white to colourless, hardness 3.5, D - 4.5
Haematite, Fe2O3
Black to cherry-red steak, hardness 5-6, D - 5.2; well crystallized haematite has a metallic lustre.
Limonite (goethite), FeO(OH).nH2O
Yellow-brown, yellow-brown streak, hardness 5, D - 4.3; limonite is largely composed of cryptocrystalline(amorphous) goethite along with adsorbed water; may exhibit botryoidal (grape) texture.
Structural Provinces of North America.
RETURN TO: