However, the results do not convince all observers. Jay Fishman,
clinical director, transplant infectious diseases, Massachusetts General
Hospital and a consultant to BioTransplant and various pharmaceutical
companies, points out that detection of infection is not clearcut.
Limits on technology available at present mean "we can't tell
directly whether or not there is infection by PERV of host tissue when
there are pig cells floating around," he says. "We impute the
fact that there is infection based on a variety of ratios of PCR
signals."
Alan Colman, director of worldwide research at PPL Therapeutics (Roslin,
UK) agrees with Fishman. "It is a model, but it's a very difficult
model to extrapolate from," says Colman. He explains that it's not
clear that the infectivity had spread to mouse cells and was not just in
the pig cells that had entered various organs. "There's a
possibility that what they were assaying was the remnants of the cells
that they originally put in those animals," he says, explaining
that completely immunocompromised mice wouldn't reject foreign tissue in
the way a normal mouse would. However, although several companies are
working on knocking out the 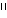 -1,3-galactosyl
transferase gene so that pig organs don't prompt acute rejection in
humans, patients would still need to be put on immunosuppressives to
avoid chronic rejection—something that puts the patient at an
increased risk for the development of retroviruses.
-1,3-galactosyl
transferase gene so that pig organs don't prompt acute rejection in
humans, patients would still need to be put on immunosuppressives to
avoid chronic rejection—something that puts the patient at an
increased risk for the development of retroviruses.
Nevertheless, Salomon's findings may not be as discouraging as they
first seem because, as Salomon himself points out, even though the mice
are severe combined immunodeficient (SCID) they are not sick and there
was no evidence for any kind of active viral spread. "All our data
suggests is that by the 2 month time point, the virus had already become
latent."
In any case, transmission from pigs to mice is a bit of a non-issue,
points out Fishman. "The fact that we may or may not be able to
infect humans or non-human primates is much closer to the area of
concern."
One of the proposed solutions to cross-species PERV transmission is
that cloning be used to generate PERV-free animals after first knocking
out or disrupting crucial PERV genes. BioTransplant's news that
peripheral blood cells from one of its line of in-bred mini-swine don't
transmit PERV A or B—2 variants that infect human cells in
culture—seems to add credence to this, even though the swine were
produced by traditional breeding methods. Although the primary data has
yet to be published in a peer-reviewed journal, Salomon is enthusiastic
about what they suggest. "The scientific impact is way, way beyond
the fact that the company accidentally has this pig," he says.