Nature 407, 27 - 30 (2000) © Macmillan Publishers Ltd.
Nature September 07, 2000
JEFFREY L. PLATT
No field of medicine has stimulated more excitement and controversy than xenotransplantation — the transplanting of animal organs and tissues into humans. The excitement stems from the possibility that transplantation could finally be extended to all patients who need it. The controversies arise from the immunological hurdles to xenotransplantation, and from the possibility that infectious agents might be passed from the animal source to the human recipient and, potentially, more broadly in the human population. In this issue are two papers (first published in electronic form three weeks ago) that speak to this excitement and controversy. On page 86, Polejaeva et al.1 describe how they have cloned pigs — a step towards surmounting the immunological hurdle. Meanwhile, on page 90, van der Laan et al.2 report that pig pancreatic islet cells, transplanted into mice, can transmit porcine endogenous retroviruses to the mice.
Pigs are ideal sources of xenotransplants because they are available in large numbers and because their organs are similar in size and nature to those of humans. Polejaeva et al.1 cloned pigs by a two-stage approach, which ultimately involved the transfer of nuclei from cultured adult cells to fertilized eggs from which the nuclei had been removed. This approach is similar to that used recently by Onishi et al.3, also to clone pigs.
Compared with breeding, cloning is not an efficient way to propagate pigs,
but it does have advantages. For example, genes might be added to, or 'knocked
out' from, the genome — modifications that are easier to achieve in cultured
cells than in whole animals. This technique might provide a way around the
rejection of 'foreign' transplanted pig organs by the human immune system. At
the moment this problem is sidestepped by damping down the recipient's immune
system. But, for example, Polejaeva et al. propose to knock out the gene
encoding the enzyme ![]() -1,3-galactosyl
transferase, which catalyses the synthesis of a sugar in pigs. This sugar is the
major molecule recognized by human antibodies that trigger xenotransplant
rejection4 (Fig.
1). This possibility seems to bring xenotransplantation closer to the
clinic, but may also provoke worries.
-1,3-galactosyl
transferase, which catalyses the synthesis of a sugar in pigs. This sugar is the
major molecule recognized by human antibodies that trigger xenotransplant
rejection4 (Fig.
1). This possibility seems to bring xenotransplantation closer to the
clinic, but may also provoke worries.
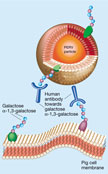 |
Figure 1 The
role of antibodies in the rejection of xenotransplants and in resistance
to porcine endogenous retroviruses (PERVs). Full legend High resolution image and legend (115k) |
Concerns about xenotransplantation stem mainly from the existence of porcine endogenous retroviruses (PERVs), which seem to be present (and harmless, if not useful) in the genome of all pigs. A retrovirus consists of a single RNA strand, enclosed in an envelope of glycoproteins derived in part from the membrane of an infected cell (Fig. 1). After infecting a cell, the RNA part of the retrovirus is copied into DNA, which inserts into the genetic content of the cell and is thus passed on to all of the cell's progeny. The DNA can also direct the formation of new viral particles. The worry is that PERVs might be transmitted first to a human transplant recipient and then more broadly in the population.
Humans have been in close contact with pigs for millennia, with blood products and tissues being exchanged by accident and, recently, by xenotransplantation4. Cultured human cells can be infected by PERVs released from cultured pig cells2, 5, but studies of 'control' humans and hundreds of patients who have received pig xenotransplants or blood plasma have not revealed even a single case in which PERVs infected human cells in the body6, 7. There are three possible explanations for this. First, PERVs may lack the necessary 'equipment' that would enable them to enter and infect human cells in vivo. Second, human cells in vivo may lack a receptor to which PERVs need to bind in order to gain entry; alternatively, they might not have the machinery needed to allow PERVs to replicate. And third, humans may have natural defences against PERVs that cultured cells do not.
The first of these explanations may now be under threat. Van der Laan et al.2 have transplanted pig pancreatic islet cells into immunodeficient mice, and found that PERVs infected several tissues — and can perhaps replicate — in these mice. Still uncertain, however, is whether human cells in vivo can be infected. Earlier results6, 7 suggest not, and one might conclude that xenotransplantation is safe. But such a conclusion would be premature, and would ignore the more important lesson to be learnt from van der Laan et al.'s work. Rodents carry a variety of viruses and retroviral elements, the genetic material of which can perhaps recombine with that of PERVs. This might give PERVs the equipment needed to for them to infect mouse cells — and perhaps even human cells — in vivo. The results reported by van der Laan et al. should serve as a warning: experimental xenotransplants in rodents could also present a risk for humans.
If PERVs have, or might acquire, the ability to infect humans, and so could be thought to pose some risk, then it becomes appropriate to ask whether we could eradicate PERVs from pigs. The method described by Polejaeva et al. 1 might offer a means of doing so. But this should not be undertaken unless the risk is certain and substantial, partly because it could have unforeseen effects on the pigs. And, because humans come into contact with pig tissues and blood in other ways (for example, through routine husbandry), the full protection of human society would require the eradication of pigs that were not modified in this way. In my opinion, the best way of determining the risk and consequences of human infection by PERVs is to conduct carefully monitored clinical trials of xenotransplants, and to treat experimental xenotransplants in rodents with the care applied to clinical xenotransplants.
Two other possible explanations for the failure so far of PERVs to infect humans are the absence of something — such as a virus receptor — in human cells, or the presence of humans' natural defences. It will be important to find out which of these possibilities is true. If the first is true, PERVs probably pose little or no threat to humans; if the second, PERVs may pose threats that have yet to be considered (Fig. 1).
Human defences against PERVs might consist of T cells, which actively fight infection, or antibodies, which are proteins that bind to foreign substances or particles and help the immune system to eliminate them. One could investigate whether T cells are involved by using van der Laan et al.'s experimental set-up to see whether PERVs in pig islet cells can infect mice with functional immune systems. This is important: although the immune systems of patients receiving xenotransplants are, at least at present, suppressed to some degree to avoid the risk of transplant rejection, it is unlikely that they are as deficient as the immune systems of van der Laan et al.'s mice.
Resistance to PERVs could also be mediated by antibodies (
Fig. 1). Pigs, mice and other lower mammals express ![]() -1,3-
galactosyl transferase, which catalyses the synthesis of galactose
-1,3-
galactosyl transferase, which catalyses the synthesis of galactose ![]() -1,3-galactose.
This sugar is found on the surface of cells from these animals. Humans and
non-human primates do not express this enzyme, but do have antibodies that
recognize its sugar product, causing the rejection of xenotransplants4.
It is tempting to propose, as Polejaeva et al.2
and others do, that techniques associated with cloning should first be used to
knock out
-1,3-galactose.
This sugar is found on the surface of cells from these animals. Humans and
non-human primates do not express this enzyme, but do have antibodies that
recognize its sugar product, causing the rejection of xenotransplants4.
It is tempting to propose, as Polejaeva et al.2
and others do, that techniques associated with cloning should first be used to
knock out ![]() -1,3-galactosyl
transferase from pigs. But PERV particles, being wrapped in pig cell membranes,
also have galactose
-1,3-galactosyl
transferase from pigs. But PERV particles, being wrapped in pig cell membranes,
also have galactose ![]() -1,3-galactose
on their surfaces. So the same antibodies can also bind to PERVs, neutralizing
the virus8. Knocking out
-1,3-galactose
on their surfaces. So the same antibodies can also bind to PERVs, neutralizing
the virus8. Knocking out ![]() -1,3-galactosyl
transferase from pigs might render viruses emerging from the xenotransplant less
susceptible to inactivation. Whether this genetic modification would endanger
humans might also be answered by thoughtful clinical studies.
-1,3-galactosyl
transferase from pigs might render viruses emerging from the xenotransplant less
susceptible to inactivation. Whether this genetic modification would endanger
humans might also be answered by thoughtful clinical studies.
| 1. | Polejaeva, I. A. et al. Nature 407, 86-90 (2000). |
| 2. | van der Laan, L. J. W. et al. Nature 407, 90-94 (2000). |
| 3. | Onishi, A. et al. Science 289, 1188-1190 (2000). Links |
| 4. | Platt, J. L. Nature 392 (suppl.), 11-17 (1998). Links |
| 5. | Patience, C., Takeuchi, Y. & Weiss, R. A. Nature Med. 3, 282-286 (1997). Links |
| 6. | Paradis, K. et al. Science 285, 1236-1241 (1999). Links |
| 7. | Heneine, W. et al. Lancet 352, 695-699 (1998). Links |
| 8. | Rother, R. P. et al. J. Exp. Med. 182, 1345-1355 (1995). Links |