CH610B Sophomore Organic II
|
Dr. Brian
Pagenkopf |
|
|
|
The Making of a
Good Leaving Group: Charge Delocalization
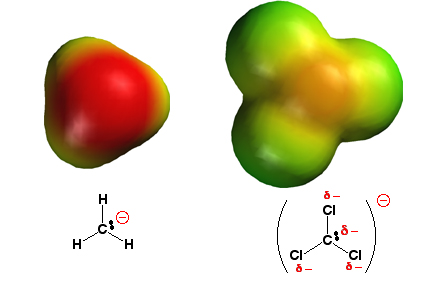
When a leaving group departs
from an intermediate, it usually carries with it a negative charge. Putting a
charge on a molecule violates a golden rule of chemistry: molecules hate
charges. However, better negative
charge delocalization makes a more stable the anion. The more stable the anion,
the better the leaving group (it costs less energy to leave). Thus, ¯:CCl3,
with the negative charge distributed partially onto the electronegative
chlorine atoms is a really good leaving group compared to ¯:CH3, where
no charge delocalization occurs.
The haloform
reaction is the first case where we’ve seen a carbon leaving group in
collapse of the tetrahedral
intermediate.
In the electrostatic potential surfaces plotted above, note how
the negative charge (red color) is localized on the carbon atom of ¯:CH3
(this is bad and makes ¯:CH3 a very bad leaving group), while there
is much less localized negative charge (the color is more yellow than red) on
the carbon atom of ¯:CCl3 (this makes ¯:CCl3 a very good
leaving group).
Sources
include
http://www.cm.utexas.edu/academic/courses/Spring2001/CH610B/Iverson/index.html