|
|
|
|
Fate of the Tetrahedral Intermediate
(for aldehyde example click here)
What
we mostly need to know about carbonyl chemistry for the entire semester can be
summarized by the few concepts
on this page.† The following three rules
are true for all carbonyl compounds:
1. the carbonyl oxygen
is basic, and will give electrons to Lewis acids
2. the carbonyl carbon
is electrophilic, and accepts electrons from and forms bonds with all sorts of nucleophiles
3. Addition of a nucleophile to
a carbonyl will give a tetrahedral intermediate,
and predicting what happens next is the same as predicting the carbonylís
chemistry.† The reaction may reverse,
stop there, or collapse to a new carbonyl compound (which may or may not go on
to react again).
Addition
to a carbonyl always gives a tetrahedral
intermediate:
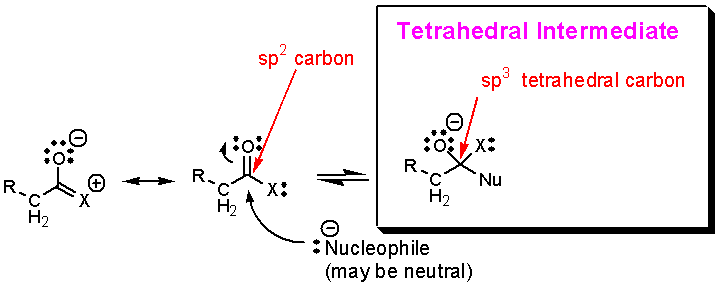
What
happens to the tetrahedral intermediate
next?† There are four possible outcomes,
and each is described below.
The
reaction may reverse back to starting materials:
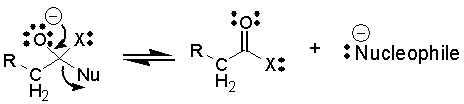
The
reaction may stop after one addition:

The
tetrahedral intermediate may collapse to a new unreactive carbonyl compound:
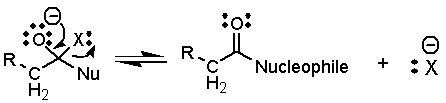
The
tetrahedral intermediate may collapse to a new carbonyl compound which reacts with the nucleophile:
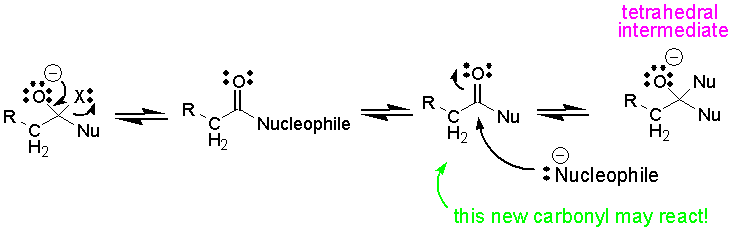
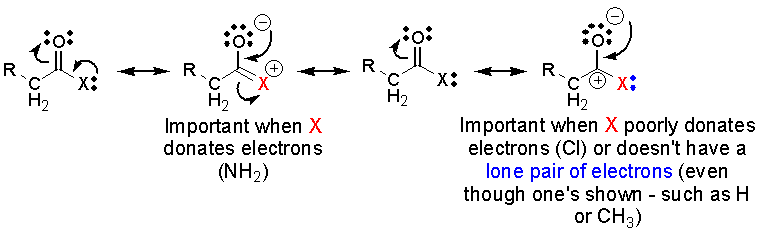
As
the ability of the group X (the X in the above structures) to donate
electron density into the carbonyl through resonance or hyperconjugation
increases:
1. the carbonyl oxygen will
become more basic
2. the carbonyl carbon becomes
less electrophilic and less reactive towards nucleophiles
The
reactivity trends of aldehydes, ketones, acids, acid chlorides, esters and
amides can be explained by understanding how X increases or decreases
reactivity.† Seeing these models of the functional groups may help you
understand their reactivity. (Hereís a
smaller pdf file of the same thing.)† Hereís a
pKa table expressing the same ideas in a more precise manner.
To predict carbonyl chemistry you must consider:
1. the strength of
the nucleophile
2. the ability of X
to serve as a leaving group
3. the ability of X
to donate electron density into the carbonyl (see discussion and models given
immediately above)
4. the reaction
conditions
5. the stability of
the proposed product under the reaction conditions
Hereís an example
with an aldehyde.